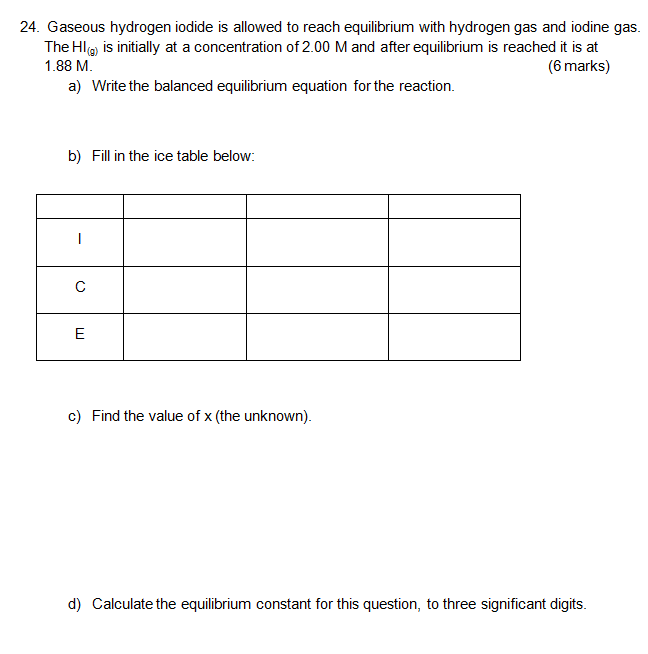
Ice Table Chemistry Khan Academy . an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. learn how to calculate the equilibrium concentrations of reactants and products from the initial concentrations and the. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. To do so, first prepare an ice (initial, change,. the spring analogy for initial and equilibrium conditions. in this video, we'll learn how to use initial concentrations along. If you're seeing this message, it means we're having trouble loading external resources on our website. Ice tables also help to solve many other types of equilibrium problems.
from socratic.org
ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice tables also help to solve many other types of equilibrium problems. To do so, first prepare an ice (initial, change,. in this video, we'll learn how to use initial concentrations along. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. learn how to calculate the equilibrium concentrations of reactants and products from the initial concentrations and the. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. If you're seeing this message, it means we're having trouble loading external resources on our website. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. the spring analogy for initial and equilibrium conditions.
Ice table..? Socratic
Ice Table Chemistry Khan Academy an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. To do so, first prepare an ice (initial, change,. Ice tables also help to solve many other types of equilibrium problems. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. If you're seeing this message, it means we're having trouble loading external resources on our website. learn how to calculate the equilibrium concentrations of reactants and products from the initial concentrations and the. in this video, we'll learn how to use initial concentrations along. the spring analogy for initial and equilibrium conditions. ice tables automatically set up and organize the variables and constants needed when calculating the unknown.
From www.youtube.com
Groups of the periodic table Periodic table Chemistry Khan Ice Table Chemistry Khan Academy To do so, first prepare an ice (initial, change,. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice tables also help to solve many other types. Ice Table Chemistry Khan Academy.
From www.youtube.com
Valence electrons and bonding ¦ Periodic table ¦ Chemistry ¦ Khan Ice Table Chemistry Khan Academy example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. the spring analogy for initial and equilibrium conditions. learn how to calculate the equilibrium concentrations of reactants and products from the initial concentrations and the.. Ice Table Chemistry Khan Academy.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing Ice Table Chemistry Khan Academy learn how to calculate the equilibrium concentrations of reactants and products from the initial concentrations and the. the spring analogy for initial and equilibrium conditions. Ice tables also help to solve many other types of equilibrium problems. If you're seeing this message, it means we're having trouble loading external resources on our website. in this video, we'll. Ice Table Chemistry Khan Academy.
From printablelibairier.z21.web.core.windows.net
Ice Table Chemistry Worksheet Ice Table Chemistry Khan Academy To do so, first prepare an ice (initial, change,. Ice tables also help to solve many other types of equilibrium problems. in this video, we'll learn how to use initial concentrations along. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. ice tables automatically set up and organize the variables and. Ice Table Chemistry Khan Academy.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps Ice Table Chemistry Khan Academy Ice tables also help to solve many other types of equilibrium problems. the spring analogy for initial and equilibrium conditions. If you're seeing this message, it means we're having trouble loading external resources on our website. To do so, first prepare an ice (initial, change,. in this video, we'll learn how to use initial concentrations along. a. Ice Table Chemistry Khan Academy.
From www.youtube.com
Equilibrium Reaction with an ICE Table Chemistry Sample Problem YouTube Ice Table Chemistry Khan Academy example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. If you're seeing this message, it means we're having trouble loading external resources on our website. learn how to calculate the equilibrium concentrations of reactants and products. Ice Table Chemistry Khan Academy.
From www.youtube.com
Calculating Ka using an ICE table YouTube Ice Table Chemistry Khan Academy example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. To do so, first prepare an ice (initial, change,. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. a compound's molar solubility in water can be calculated. Ice Table Chemistry Khan Academy.
From www.youtube.com
The periodic table classification of elements Chemistry Khan Ice Table Chemistry Khan Academy To do so, first prepare an ice (initial, change,. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. learn how to calculate the equilibrium concentrations of. Ice Table Chemistry Khan Academy.
From www.youtube.com
ICE tables and equilibrium calculations Real Chemistry YouTube Ice Table Chemistry Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. the spring analogy for initial and equilibrium conditions. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating. Ice Table Chemistry Khan Academy.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing Ice Table Chemistry Khan Academy Ice tables also help to solve many other types of equilibrium problems. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. the spring analogy for initial and equilibrium conditions. If you're seeing this message, it means we're having trouble loading external resources on. Ice Table Chemistry Khan Academy.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership Ice Table Chemistry Khan Academy Ice tables also help to solve many other types of equilibrium problems. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. If you're seeing this message, it means we're having trouble loading external resources on our website. in this video, we'll learn how to use initial concentrations along. ice tables automatically. Ice Table Chemistry Khan Academy.
From www.youtube.com
Transition Metals Periodic table Chemistry Khan Academy YouTube Ice Table Chemistry Khan Academy To do so, first prepare an ice (initial, change,. If you're seeing this message, it means we're having trouble loading external resources on our website. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. the spring analogy for initial and equilibrium conditions. Ice tables also help to solve many other types of. Ice Table Chemistry Khan Academy.
From www.tunggaksemi.eu.org
Khan Academy Chemistry Class 11 Tunggak Semi Ice Table Chemistry Khan Academy a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. If you're seeing this message, it means we're having trouble loading external resources on our website. To do so, first prepare an ice (initial, change,. the spring analogy for initial and equilibrium conditions. learn how to calculate the equilibrium concentrations of reactants. Ice Table Chemistry Khan Academy.
From www.youtube.com
Perfect Squares ICE Table Equilibrium Calculations YouTube Ice Table Chemistry Khan Academy example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. the spring analogy for initial and equilibrium conditions. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. learn how to calculate the equilibrium concentrations of reactants and products from the initial concentrations and the. . Ice Table Chemistry Khan Academy.
From z-cm.blogspot.com
Ice Table Chem Decoration Examples Ice Table Chemistry Khan Academy in this video, we'll learn how to use initial concentrations along. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. a compound's molar solubility in water. Ice Table Chemistry Khan Academy.
From www.youtube.com
ICE Tables Given K but NO Equilibrium Concentrations YouTube Ice Table Chemistry Khan Academy in this video, we'll learn how to use initial concentrations along. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Ice tables also help to solve many. Ice Table Chemistry Khan Academy.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing Ice Table Chemistry Khan Academy ice tables automatically set up and organize the variables and constants needed when calculating the unknown. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. a. Ice Table Chemistry Khan Academy.
From www.youtube.com
Ksp calculations ICE table shortcuts YouTube Ice Table Chemistry Khan Academy ice tables automatically set up and organize the variables and constants needed when calculating the unknown. a compound's molar solubility in water can be calculated from its kₛₚ value at 25°c. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Ice tables. Ice Table Chemistry Khan Academy.